Spatial statistics in R#
Extra material
This page provides additional material on using R for spatial statistics. This is rather specialised set of techniques that are occasionally used in Masters projects. This practical provides an overview of some of the problems of fitting statistical models to spatial data using R and is provided as an introduction for students that need to use these techniques.
The practical covers a lot of ground but do not panic: the intention is to show some options that are available for spatial models in R, so you can assess what options are available and how they differ.
There are a lot of other resources out there to provide more detail and I highly recommend this:
https://rspatial.org/raster/analysis/index.html
Required packages#
As with species distribution mdelling, there are many packages used in spatial modelling. There is an intimidating complete list of topics and packages here:
https://CRAN.R-project.org/view=Spatial
We will need to load the following packages. Remember to read this guide on setting up packages on your computer before running these practicals on your own machine.
library(ncf)
library(raster)
library(sf)
library(spdep)
library(SpatialPack)
library(spatialreg)
library(nlme)
library(spgwr)
library(spmoran)
The dataset#
We will use some data taken from a paper that looked at trying to predict what limits species ranges:
McInnes, L., Purvis, A., & Orme, C. D. L. (2009). Where do species’ geographic ranges stop and why? Landscape impermeability and the Afrotropical avifauna. Proceedings of the Royal Society Series B - Biological Sciences, 276(1670), 3063-3070. http://doi.org/10.1098/rspb.2009.0656
We won’t actually be looking at range edges but we’re going to use four variables taken from the data used in this paper. The data is all saved as GeoTIFF files, so we’re starting with raster data. The files cover the Afrotropics and are all projected in the Behrmann equal area projection. This is a cylindrical equal area projection with a latitude of true scale of 30°. What that means is that distances on the projected map correspond to distances over the surface of the Earth at ±30° of latitude.
That is the reason for the odd resolution of this data: 96.48627 km. The circumference of the Earth at ±30° - the length of the parallels at ±30° - is ~ 34735.06 km and this resolution splits the map into 360 cells giving a resolution comparable to a degree longitude at 30° N. Unlike a one degree resolution grid, however, these grid cells all cover an equal area on the ground (\(96.48627 \times 96.48627 = 9309.6 \text{km}^2\), roughly the land area of Cyprus).
The variables for each grid cell are:
the avian species richness across the Afrotropics,
the mean elevation,
the average annual temperature, and
the average annual actual evapotranspiration.
Loading the data#
# load the four variables from their TIFF files
rich <- raster('data/spatial_models/avian_richness.tif')
aet <- raster('data/spatial_models/mean_aet.tif')
temp <- raster('data/spatial_models/mean_temp.tif')
elev <- raster('data/spatial_models/elev.tif')
It is always a good idea to look at the details of the data. One key skill in being a good scientist and statistician is in looking at data and models and saying:
Uh, that doesn’t make any sense, something has gone wrong.
So we will quickly use some techniques to explore the data we have just loaded.
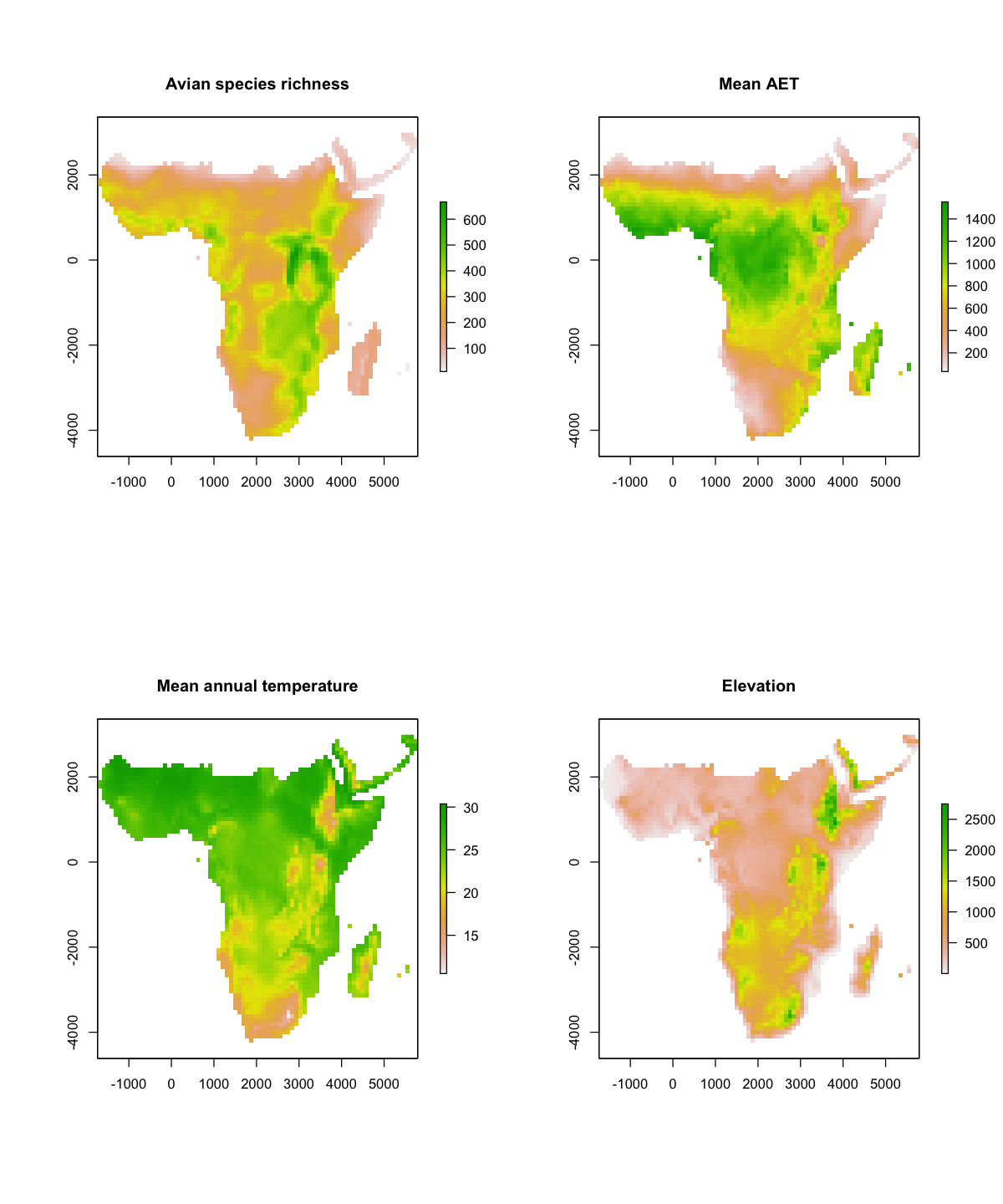
We will look at the summary of the richness data. That shows us the dimensions of the data, resolution, extent and coordinate reference system and the range of the data: between 10 and 667 species per cell.
# Look at the details of the richness data
print(rich)
class : RasterLayer
dimensions : 75, 78, 5850 (nrow, ncol, ncell)
resolution : 96.48627, 96.48627 (x, y)
extent : -1736.753, 5789.176, -4245.396, 2991.074 (xmin, xmax, ymin, ymax)
crs : +proj=cea +lat_ts=30 +lon_0=0 +x_0=0 +y_0=0 +datum=WGS84 +units=km +no_defs
source : avian_richness.tif
names : avian_richness
values : 10, 667 (min, max)
We can also plot maps the variables and think about the spatial patterns in each.
par(mfrow=c(2,2))
plot(rich, main='Avian species richness')
plot(aet, main='Mean AET')
plot(temp, main='Mean annual temperature')
plot(elev, main='Elevation')
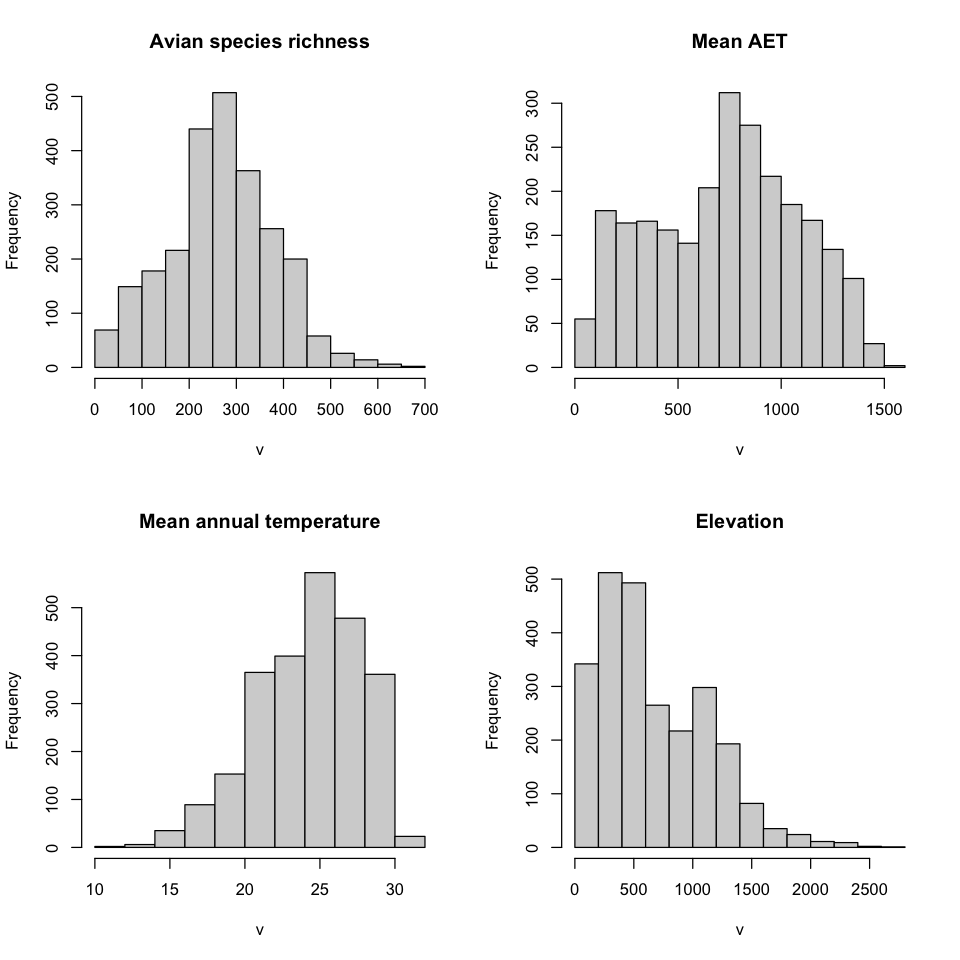
We can use R to explore this data a bit further. We can use the hist() function to
plot the distribution of the values in each variable, not just look at the minimum and
maximum.
Formatting the data as a data frame#
We now have the data as rasters but some of the methods require the values in a data frame. We’ll need to use two commands:
First, stack() allows us to concatenate the four rasters into a single object. Note
that this only works because all of these rasters have the same projection, extent and
resolution. In your own use, you would need to use GIS to set up your data so it can be
stacked like this.
# Stack the data
data_stack <- stack(rich, aet, elev, temp)
print(data_stack)
class : RasterStack
dimensions : 75, 78, 5850, 4 (nrow, ncol, ncell, nlayers)
resolution : 96.48627, 96.48627 (x, y)
extent : -1736.753, 5789.176, -4245.396, 2991.074 (xmin, xmax, ymin, ymax)
crs : +proj=cea +lat_ts=30 +lon_0=0 +x_0=0 +y_0=0 +datum=WGS84 +units=km +no_defs
names : avian_richness, mean_aet, elev, mean_temp
min values : 10.0000, 32.0600, 1.0000, 10.5246
max values : 667.0000, 1552.6500, 2750.2800, 30.3667
Second, as() allows us to convert an R object from one format to another. Here we
convert to the SpatialPixelDataFrame format from the sp package. This is a useful
format because it works very like a data frame, but identifies the geometry as pixels.
Note that the names of the variables in the data frame have been set from the original
TIFF filenames, not our variable names in R.
data_spdf <- as(data_stack, 'SpatialPixelsDataFrame')
summary(data_spdf)
Object of class SpatialPixelsDataFrame
Coordinates:
min max
x -1736.753 5789.176
y -4245.396 2991.074
Is projected: TRUE
proj4string :
[+proj=cea +lat_ts=30 +lon_0=0 +x_0=0 +y_0=0 +datum=WGS84 +units=km
+no_defs]
Number of points: 2484
Grid attributes:
cellcentre.offset cellsize cells.dim
s1 -1688.510 96.48627 78
s2 -4197.153 96.48627 75
Data attributes:
avian_richness mean_aet elev mean_temp
Min. : 10.0 Min. : 32.06 Min. : 1.0 Min. :10.52
1st Qu.:202.0 1st Qu.: 439.70 1st Qu.: 317.7 1st Qu.:21.83
Median :269.5 Median : 754.59 Median : 548.8 Median :24.71
Mean :268.4 Mean : 732.07 Mean : 672.0 Mean :24.25
3rd Qu.:341.0 3rd Qu.: 996.60 3rd Qu.:1023.2 3rd Qu.:27.15
Max. :667.0 Max. :1552.65 Max. :2750.3 Max. :30.37
We can also that into a sf object, which we have been using in previous practicals.
These differ in how they represent the geometry:
SpatialPixelDataFrame: this holds the data as values in pixels, so ‘knows’ that the values represents an area.sf: the default conversion here holds the data as values at a point.
data_sf <- st_as_sf(data_spdf)
print(data_sf)
Simple feature collection with 2484 features and 4 fields
Geometry type: POINT
Dimension: XY
Bounding box: xmin: -1736.753 ymin: -4245.396 xmax: 5789.176 ymax: 2991.074
Projected CRS: +proj=cea +lat_ts=30 +lon_0=0 +x_0=0 +y_0=0 +datum=WGS84 +units=km +no_defs
First 10 features:
avian_richness mean_aet elev mean_temp geometry
1 55 157.4988 619.990 25.5596 POINT (5451.474 2942.831)
2 52 117.5050 353.648 26.7782 POINT (5547.96 2942.831)
3 44 93.1765 270.054 27.3340 POINT (5644.447 2942.831)
4 45 86.4079 1043.670 24.9595 POINT (3907.694 2846.345)
5 38 136.8711 353.393 27.2728 POINT (5451.474 2846.345)
6 50 99.2078 773.086 24.9603 POINT (5547.96 2846.345)
7 49 110.1031 610.546 25.6667 POINT (5644.447 2846.345)
8 38 122.4231 820.173 25.0142 POINT (5740.933 2846.345)
9 58 70.4857 294.331 28.0947 POINT (3811.208 2749.859)
10 59 115.8227 1064.230 24.9419 POINT (3907.694 2749.859)
# Get the cell resolution
cellsize <- res(rich)[[1]]
# Make the template polygon
template <- st_polygon(list(matrix(c(-1,-1,1,1,-1,-1,1,1,-1,-1), ncol=2) * cellsize / 2))
# Add each of the data points to the template
polygon_data <- lapply(data_sf$geometry, function(pt) template + pt)
data_poly <- st_sf(avian_richness = data_sf$avian_richness,
geometry=polygon_data)
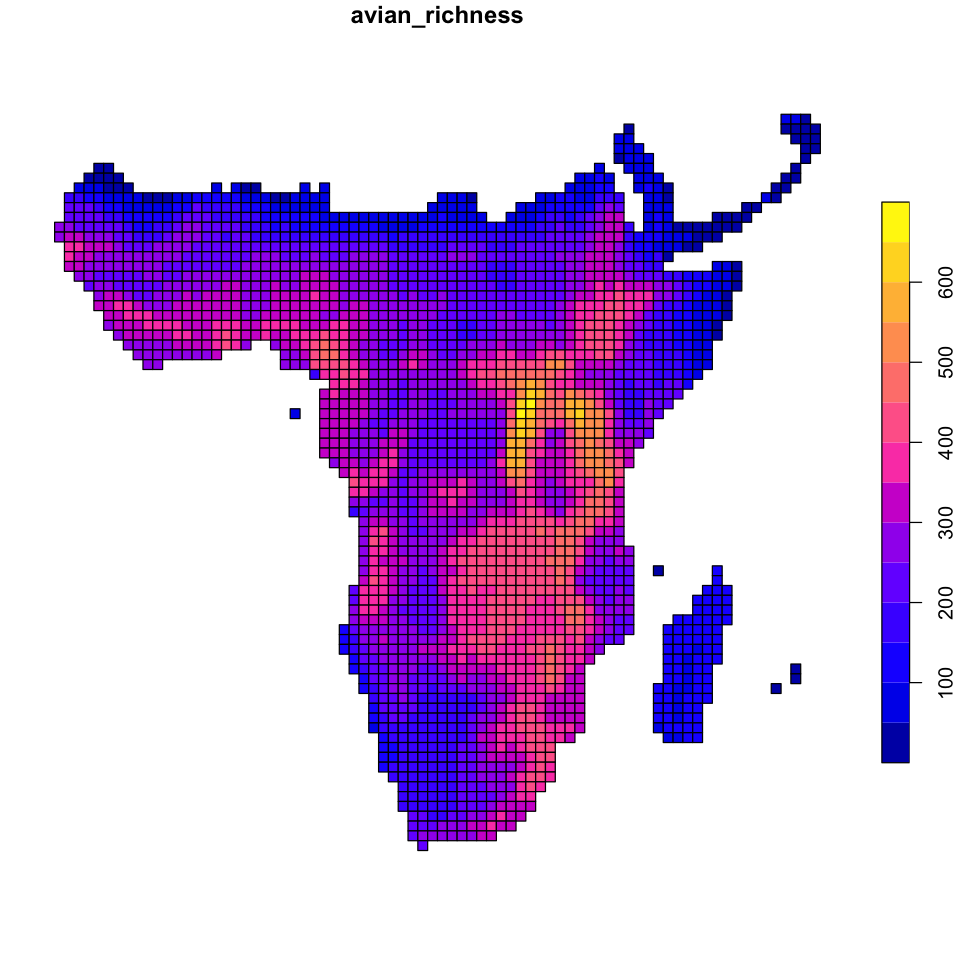
plot(data_poly['avian_richness'])
More data exploration#
We now have new data structures that have spatial information and matches up the different variables across locations.
We can still plot the data as a map and the images below show the difference between the
SpatialPixelsDataFrame and the sf version of the data. The code has lots of odd
options: this combination of settings avoids each plot command insisting on the layout
it wants to use and lets us
control the layout of the plots.
# Plot the richness data as point data
layout(matrix(1:3, ncol=3), widths=c(5,5,1))
plot(data_spdf['avian_richness'], col=hcl.colors(20), what='image')
plot(data_sf['avian_richness'], key.pos=NULL, reset=FALSE, main='',
pal=hcl.colors, cex=0.7, pch=20)
plot(data_spdf['avian_richness'], col=hcl.colors(20), what='scale')
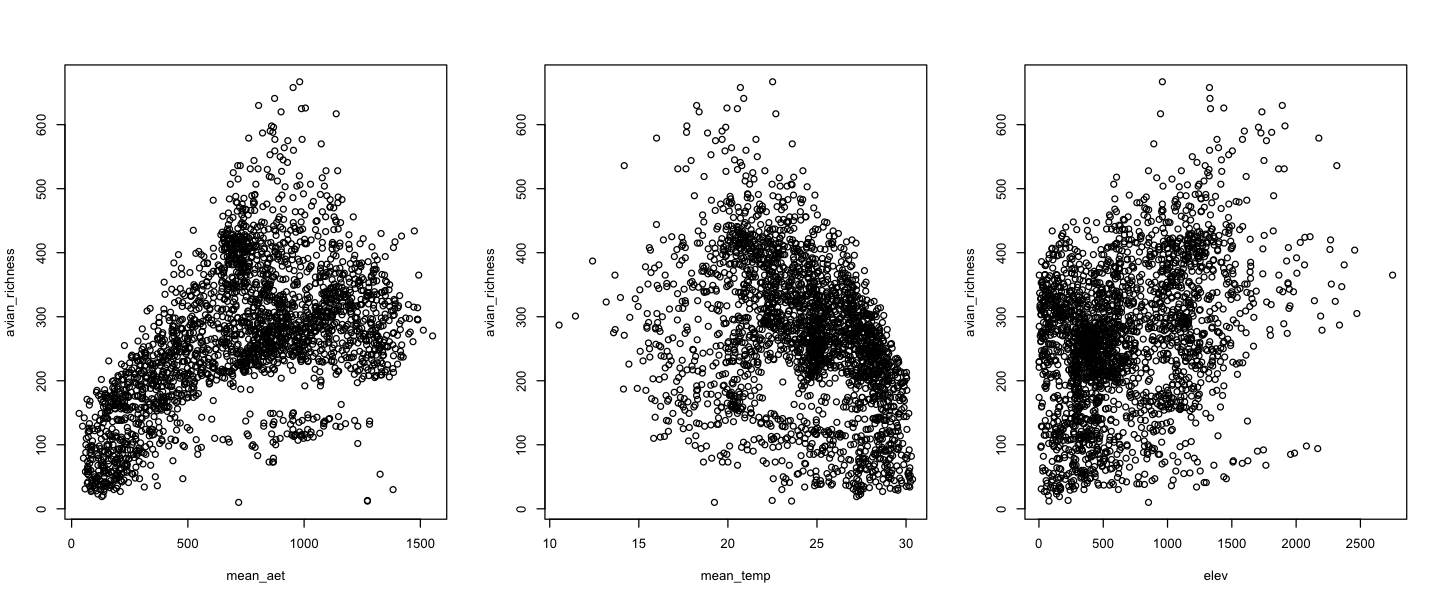
We can also plot the variables against each other, by treating the new object as a data frame:
Correlations and spatial data#
A correlation coefficient is a standardised measure between -1 and 1 showing how much observations of two variables tend to co-vary. For a positive correlation, both variables tend to have high values in the same locations and low values in the same locations. Negative correlations show the opposite. Once you’ve calculated a correlation, you need to assess how strong that correlation is given the amount of data you have: it is easy to get large \(r\) values in small datasets by chance.
However, correlation coefficients assume that the data points are independent and this is not true for spatial data. Nearby data points tend to be similar: if you are in a warm location, surrounding areas will also tend to be warm. One relatively simple way of removing this non-independence is to calculate the significance of tests as if you had fewer data points.
We will use this approach to compare standard measures of correlation to spatially corrected ones. We need to load a new set of functions that implement a modification to the correlation test that accounts for spatial similarity, described in this paper. The modified test does not change the correlation statistic itself but calculates a new effective sample size and uses this in calculating the \(F\) statistic.
# Use the modified.ttest function from SpatialPack
temp_corr <- modified.ttest(x=data_sf$avian_richness, y=data_sf$mean_temp,
coords=st_coordinates(data_sf))
print(temp_corr)
Corrected Pearson's correlation for spatial autocorrelation
data: x and y ; coordinates: X and Y
F-statistic: 5.1886 on 1 and 32.263 DF, p-value: 0.0295
alternative hypothesis: true autocorrelation is not equal to 0
sample correlation: -0.3722
Other variables
Use this code to look at how the other explanatory variables with bird richness. It is also worth looking at correlations between the explanatory variables!
Neighbourhoods#
One of the core concepts in many spatial statistical methods is the neighbourhood of
cells. The neighbourhood of a cell defines a set of other cells that are connected to
the focal cell, often with a given weight. The neighbourhood is one way of providing
spatial structure to the statistical methods and there are many options for doing this.
The spdep package provides a good guide (vignette('nb')) on the details, but we will
look at two functions: dnearneigh and knearneigh.
dnearneigh#
We can use this function to find which cells are within a given distance of a focal point. We can also put a minimum distance to exclude nearby, but we will keep that at zero here. Working with raster data,where points are on an even grid, we can use this function to generate the Rook and Queen move neighbours. To do that we need to get the resolution of the raster and use that to set appropriate distances.
# Give dnearneigh the coordinates of the points and the distances to use
rook <- dnearneigh(data_sf, d1=0, d2=cellsize)
queen <- dnearneigh(data_sf, d1=0, d2=sqrt(2) * cellsize)
If we look at those in a bit more details we can see that they are very similar but -
surprise! - there are more cells linked as neighbours in queen. Using head allows us
to see the row numbers in our data frame that are neighbours. We can also look at the
number of neighbours in each set (the cardinality, hence the function name card). If
we store that in our data_sf data frame, we can then visualise the number of
neighbours.
print(rook)
head(rook, n=3)
Neighbour list object:
Number of regions: 2484
Number of nonzero links: 6010
Percentage nonzero weights: 0.09740277
Average number of links: 2.419485
4 regions with no links:
159 1311 1817 2204
-
- 2
- 5
-
- 1
- 6
- 7
print(queen)
head(queen, n=3)
Neighbour list object:
Number of regions: 2484
Number of nonzero links: 18728
Percentage nonzero weights: 0.3035206
Average number of links: 7.539452
3 regions with no links:
1311 1817 2204
-
- 2
- 5
- 6
-
- 1
- 3
- 5
- 6
- 7
-
- 2
- 6
- 7
- 8
# Store the neighbourhood cardinalities in data_sf
data_sf$card_rook <- card(rook)
data_sf$card_queen <- card(queen)
# Look at the count of rook and queen neighbours for each point
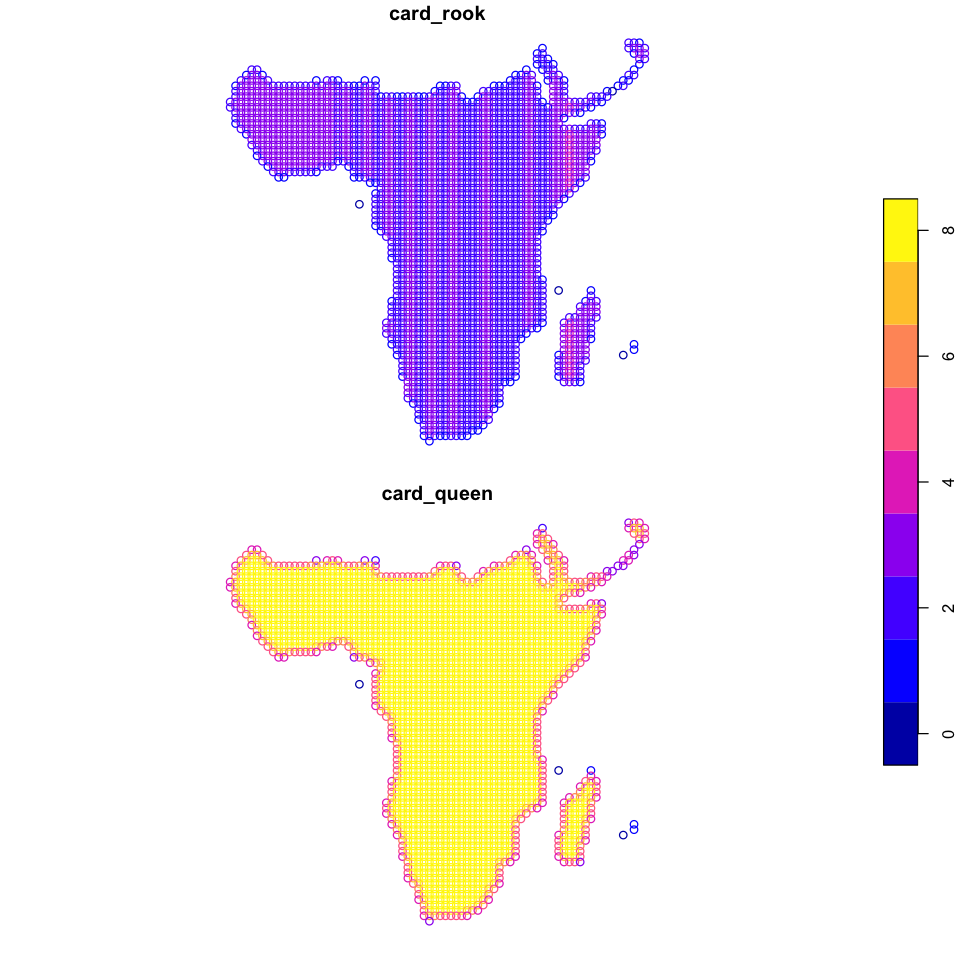
plot(data_sf[c('card_rook', 'card_queen')], key.pos=4)
That does not look correct - we should not be seeing those stripes in central Africa with only two or three rook neighbours. The reason for this is using exactly the resolution as our distance: minor rounding differences can lead to distance based measures going wrong, so it is once again always a good idea to plot your data and check!
Fix the neighbours
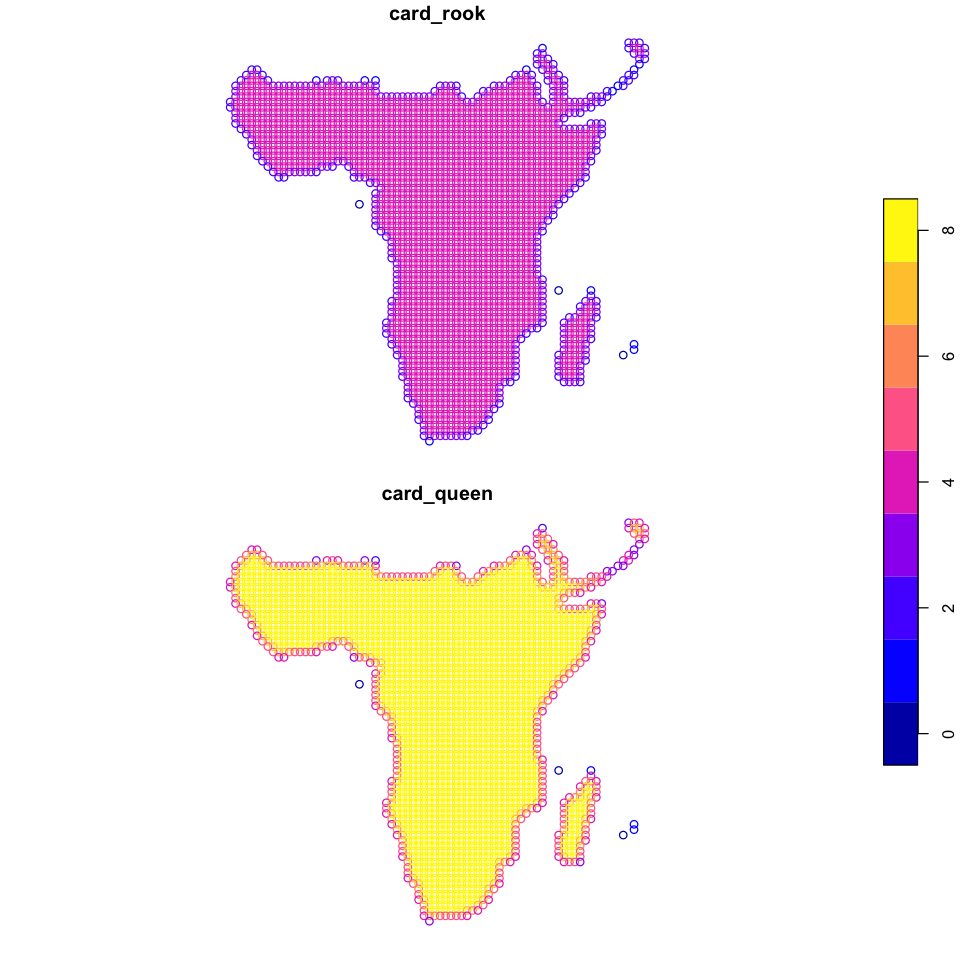
A simple solution here is just to make the distance very slightly larger. Do this and
recreate the rook and queen neighbours. The new version should look like the figure
below.
Show code cell source
# Recreate the neighbour adding 1km to the distance
rook <- dnearneigh(data_sf, d1=0, d2=cellsize + 1)
queen <- dnearneigh(data_sf, d1=0, d2=sqrt(2) * cellsize + 1)
data_sf$card_rook <- card(rook)
data_sf$card_queen <- card(queen)
plot(data_sf[c('card_rook', 'card_queen')], key.pos=4)
knearneigh#
One thing to note in the details of rook and queen are the bits that say:
3 regions with no links:
1311 1817 2204
These are points that have no other point within the provided distance. These are
islands: São Tomé and Principe, Comoros, and Réunion - it just happens that Mauritius
falls into two cells and so has itself as a neighbour. The knearneigh function ensures
that all points have the same number of neighbours by looking for the \(k\) closest
locations. You end up with a matrix
knn <- knearneigh(data_sf, k=8)
# We have to look at the `nn` values in `knn` to see the matrix of neighbours
head(knn$nn, n=3)
| 5 | 2 | 6 | 3 | 11 | 7 | 12 | 8 |
| 6 | 1 | 3 | 5 | 7 | 11 | 12 | 8 |
| 7 | 2 | 8 | 6 | 12 | 1 | 13 | 11 |
Spatial weights#
The neighbourhood functions just give us sets of neighbours, and most spatial modelling
functions require weights to be assigned to neighbours. In spdep, we need to use
nb2listw to take our plain sets of neighbours and make them into weighted neighbour
lists.
queen <- nb2listw(queen)
Error in nb2listw(queen): Empty neighbour sets found
Traceback:
1. nb2listw(queen)
2. stop("Empty neighbour sets found")
That didn’t work! The error message is fairly clear (for R) - we cannot create weighted lists for locations with no neighbours. We have two choices here:
Remove the points with no neighbours - given these are isolated offshore islands, this seems reasonable.
Use a neighbourhood system in which they are not isolated. We already have this using
knearneigh, but you do have to ask yourself if a model that arbitrarily links offshore islands is realistic.
More data cleaning#
It would be easy to use subset on data_sf to remove cells with zero neighbours, but
we really should remove Mauritius as well (two cells with cardinality of 1).
Unfortunately there is a coastal cell with a rook cardinality of 1 in the north of
Madagascar, and that probably is reasonable to include! So, we will use a GIS operation.
# Polygon covering Mauritius
mauritius <- st_polygon(list(matrix(c(5000, 6000, 6000, 5000, 5000,
0, 0, -4000, -4000, 0),
ncol=2)))
mauritius <- st_sfc(mauritius, crs=crs(data_sf))
# Remove the island cells with zero neighbours
data_sf <- subset(data_sf, card_rook > 0)
# Remove Mauritius
data_sf <- st_difference(data_sf, mauritius)
Warning message:
“attribute variables are assumed to be spatially constant throughout all geometries”
We do now need to recalculate our neighbourhood objects to use that reduced set of locations.
rook <- dnearneigh(data_sf, d1=0, d2=cellsize + 1)
queen <- dnearneigh(data_sf, d1=0, d2=sqrt(2) * cellsize + 1)
data_sf$card_rook <- card(rook)
data_sf$card_queen <- card(queen)
knn <- knearneigh(data_sf, k=8)
Calculating weights#
There are several weighting styles and choosing different strategies obviously affects
the resulting statistics. One option is binary weights (cells are either connected
or not) but there are other options. Here we will use the default row standardised
(style='W'), which just means that the neighbours of location all get the same weight
but the sum of neighbour weights for location is always one.
Note that knn needs to be converted to the same format (nb) as the rook and queen
neighbourhoods first.
rook <- nb2listw(rook, style='W')
queen <- nb2listw(queen, style='W')
knn <- nb2listw(knn2nb(knn), style='W')
Spatial autocorrelation metrics#
Spatial autocorrelation describes the extent to which points that are close together in space show similar values. It can be measured globally and locally - note that global here means the whole of the dataset and not the whole of the Earth.
Global spatial autocorrelation#
There are a number of statistics that quantify the level of global spatial autocorrelation in a dataset. These statistics test if the dataset as a whole shows spatial autocorrelation.
We will be looking at the commonly used Moran’s \(I\) and Geary’s \(C\) statistics
Moran’s \(I\) takes values between -1 and 1, with the central value of 0 showing that there is no spatial autocorrelation. Values close to -1 show strong negative autocorrelation, which is the unusual case that nearby cells have unexpectedly different values and values close to 1 show that nearby cells have unexpectedly similar values.
Geary’s \(C\) scales the other way around. It is not perfectly correlated with Moran’s \(I\) but closely related.
moran_avian_richness <- moran.test(data_sf$avian_richness, rook)
print(moran_avian_richness)
Moran I test under randomisation
data: data_sf$avian_richness
weights: rook
Moran I statistic standard deviate = 64.66, p-value < 2.2e-16
alternative hypothesis: greater
sample estimates:
Moran I statistic Expectation Variance
0.9499254829 -0.0004035513 0.0002160130
geary_avian_richness <- geary.test(data_sf$avian_richness, rook)
print(geary_avian_richness)
Geary C test under randomisation
data: data_sf$avian_richness
weights: rook
Geary C statistic standard deviate = 64.254, p-value < 2.2e-16
alternative hypothesis: Expectation greater than statistic
sample estimates:
Geary C statistic Expectation Variance
0.0468078533 1.0000000000 0.0002200701
Variables and neighbours
Look to see if the different neighbourhood schemes increase or decrease spatial autocorrelation. Do all of the other three variables show significant spatial autocorrelation?
Local spatial autocorrelation#
We can also look at local indicators of spatial autocorrelation (LISA) to show areas of stronger or weaker similarity. These calculate a similar statistic but only within the neighbourhood around each cell and report back the calculated value for each cell, which we can then map.
The localmoran function outputs a matrix of values. The columns contain observed \(I\),
the expected value, variance, \(z\) statistics and \(p\) value and the rows contain the
location specific measures of each variable, so we can load them into data_sf.
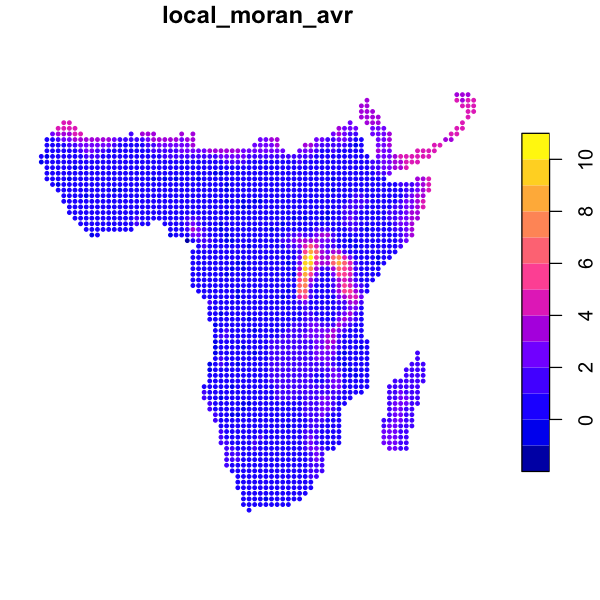
local_moran_avr <- localmoran(data_sf$avian_richness, rook)
data_sf$local_moran_avr <- local_moran_avr[, 'Ii'] # Observed Moran's I
plot(data_sf['local_moran_avr'], cex=0.6, pch=20)
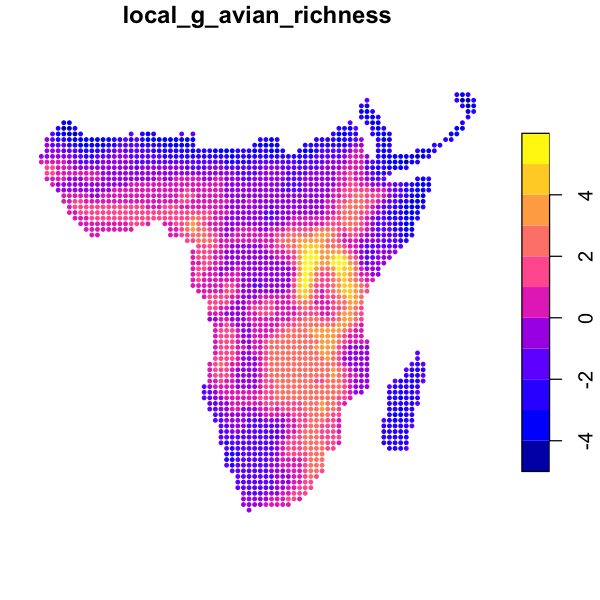
The similar function localG just calculates a \(z\) statistic showing strength of local
autocorrelation.
data_sf$local_g_avian_richness <- localG(data_sf$avian_richness, rook)
plot(data_sf['local_g_avian_richness'], cex=0.6, pch=20)
Exploring local spatial autocorrelation
As we might predict, avian richness shows strong positive spatial autocorrelation, which seems to be particularly strong in the mountains around Lake Victoria. Try these measures out on the other variables and neighbourhoods.
Autoregressive models#
Our definition of a set of neighbours allows us to fit a spatial autoregressive (SAR) model. This is a statistical model that predicts the value of a response variable in a cell using the predictor variables and values of the response variable in neighbouring cells. This is why they are called autoregressive models: they fit the response variable partly as a response to itself.
They come in many different possible forms. This is a great paper explaining some of the different forms with some great appendices including example R code:
Kissling, W.D. and Carl, G. (2008), Spatial autocorrelation and the selection of simultaneous autoregressive models. Global Ecology and Biogeography, 17: 59-71. doi:10.1111/j.1466-8238.2007.00334.x
# Fit a simple linear model
simple_model <- lm(avian_richness ~ mean_aet + elev + mean_temp, data=data_sf)
summary(simple_model)
Call:
lm(formula = avian_richness ~ mean_aet + elev + mean_temp, data = data_sf)
Residuals:
Min 1Q Median 3Q Max
-286.82 -53.00 -1.53 51.95 324.58
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 198.276692 21.062878 9.414 < 2e-16 ***
mean_aet 0.178391 0.004665 38.238 < 2e-16 ***
elev 0.073595 0.005422 13.573 < 2e-16 ***
mean_temp -4.508362 0.713118 -6.322 3.05e-10 ***
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
Residual standard error: 81.24 on 2475 degrees of freedom
Multiple R-squared: 0.4665, Adjusted R-squared: 0.4659
F-statistic: 721.4 on 3 and 2475 DF, p-value: < 2.2e-16
# Fit a spatial autoregressive model: this is much slower and can take minutes to calculate
sar_model <- errorsarlm(avian_richness ~ mean_aet + elev + mean_temp,
data=data_sf, listw=queen)
summary(sar_model)
Call:errorsarlm(formula = avian_richness ~ mean_aet + elev + mean_temp,
data = data_sf, listw = queen)
Residuals:
Min 1Q Median 3Q Max
-218.57762 -9.64130 -0.54812 9.09747 109.78562
Type: error
Coefficients: (asymptotic standard errors)
Estimate Std. Error z value Pr(>|z|)
(Intercept) 44.2663338 64.8564135 0.6825 0.49490
mean_aet 0.0581636 0.0070908 8.2027 2.220e-16
elev 0.0485815 0.0067335 7.2149 5.396e-13
mean_temp 3.0505866 1.2710584 2.4000 0.01639
Lambda: 0.99316, LR test value: 6447.6, p-value: < 2.22e-16
Asymptotic standard error: 0.0017541
z-value: 566.2, p-value: < 2.22e-16
Wald statistic: 320580, p-value: < 2.22e-16
Log likelihood: -11193.01 for error model
ML residual variance (sigma squared): 364.6, (sigma: 19.095)
Number of observations: 2479
Number of parameters estimated: 6
AIC: NA (not available for weighted model), (AIC for lm: 28844)
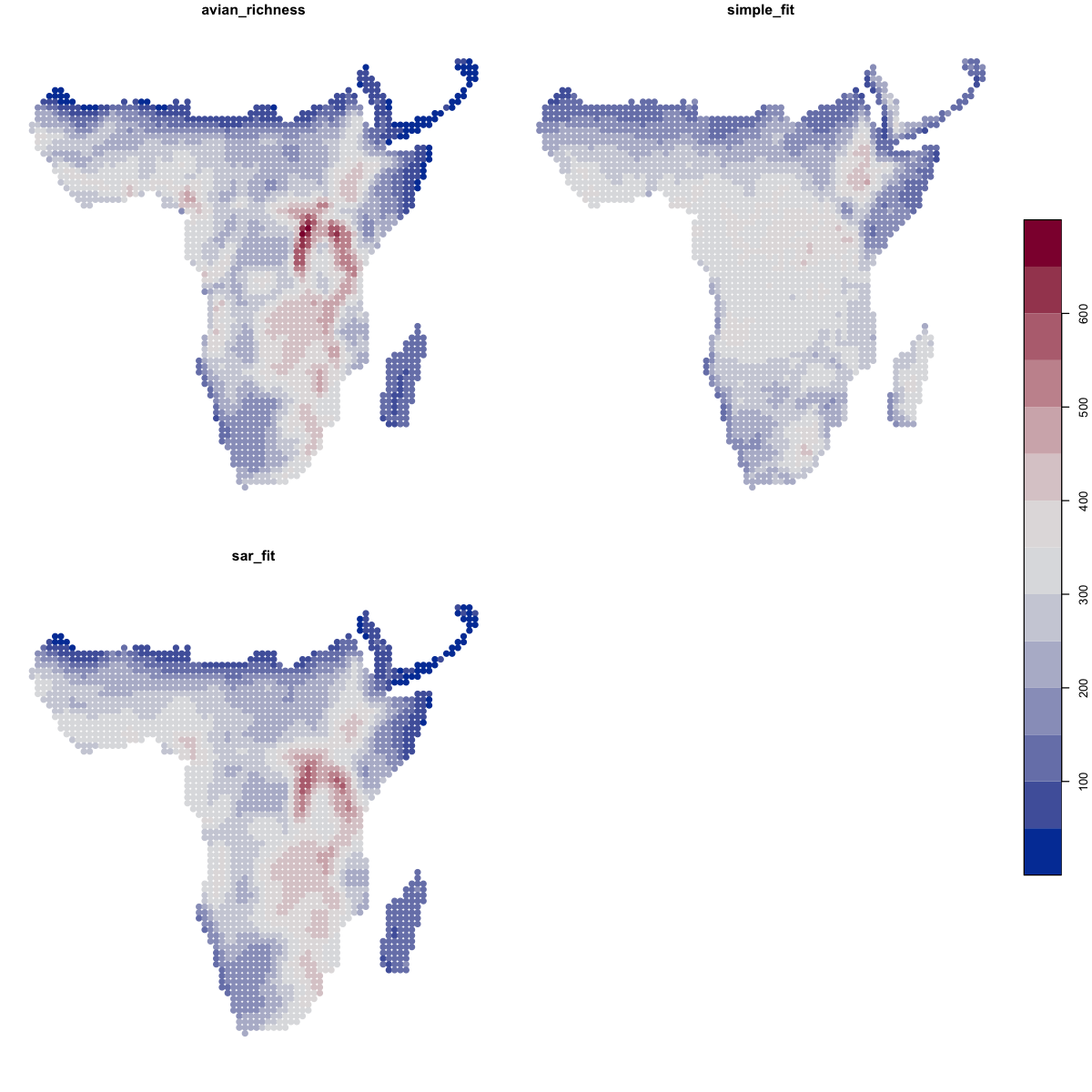
We can then look at the predictions of those models. We can extract the predicted values for each point and put them into our spatial data frame and then map them.
# extract the predictions from the model into the spatial data frame
data_sf$simple_fit <- predict(simple_model)
data_sf$sar_fit <- predict(sar_model)
# Compare those two predictions with the data
plot(data_sf[c('avian_richness','simple_fit','sar_fit')],
pal=function(n) hcl.colors(n, pal='Blue-Red'), key.pos=4, pch=19)
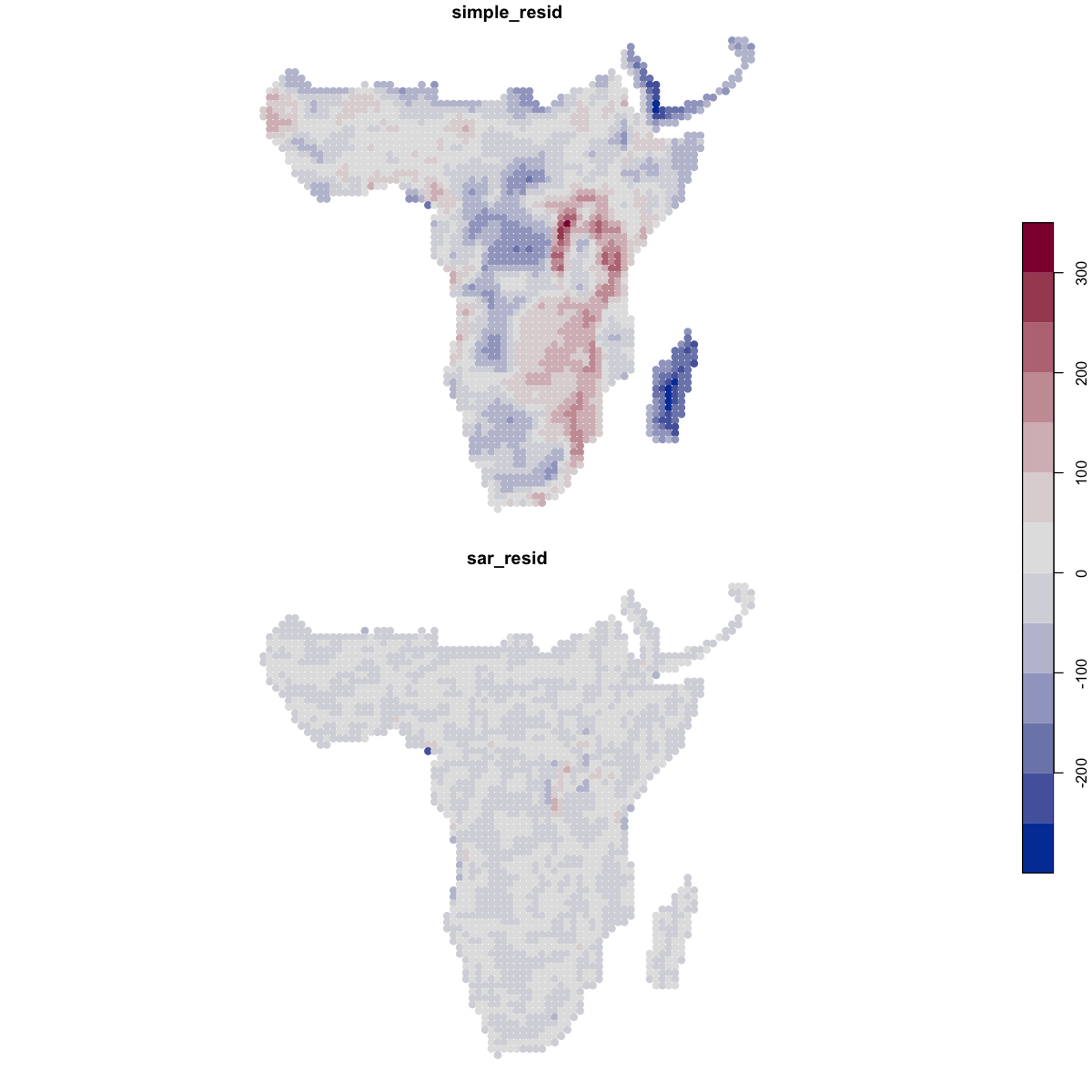
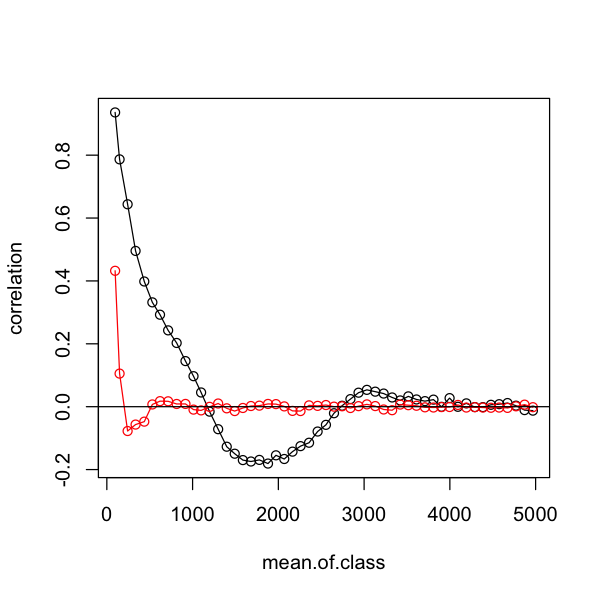
We can also look at the residuals of those models - the differences between the prediction and the actual values - to highlight where the models aren’t working well. The residuals from the SAR are far better behaved.
# extract the residuals from the model into the spatial data frame
data_sf$simple_resid <- residuals(simple_model)
data_sf$sar_resid <- residuals(sar_model)
plot(data_sf[c('simple_resid', 'sar_resid')],
pal=function(n) hcl.colors(n, pal='Blue-Red'), key.pos=4, pch=19)
Correlograms#
Correlograms are another way of visualising spatial autocorrelation. They show how the correlation within a variable changes as the distance between pairs of points being compared increases. To show this, we need the coordinates of the spatial data and the values of a variable at each point.
# add the X and Y coordinates to the data frame
data_xy <- data.frame(st_coordinates(data_sf))
data_sf$x <- data_xy$X
data_sf$y <- data_xy$Y
# calculate a correlogram for avian richness: a slow process!
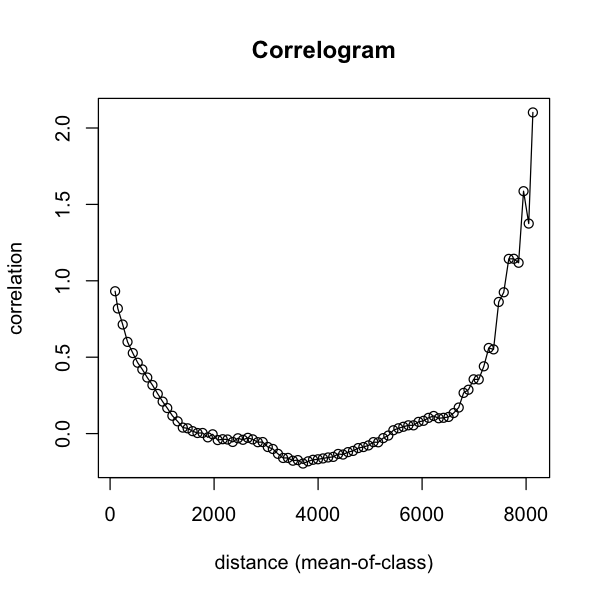
rich.correlog <- with(data_sf, correlog(x, y, avian_richness, increment=cellsize, resamp=0))
plot(rich.correlog)
To explain that a bit further: we take a focal point and then make pairs of the value of
that point and all other points. These pairs are assigned to bins based on how far apart
the points are: the increment is the width of those bins in map units. Once we’ve done
this for all points - yes, that is a lot of pairs! - we calculate the correlations
between the sets of pairs in each bin. Each bin has a mean distance among the points in
that class.
We can get the significance of the correlations at each distance by resampling the data,
but it is a very slow process, which is why the correlograms here have been set not to
do any resampling (resamp=0).
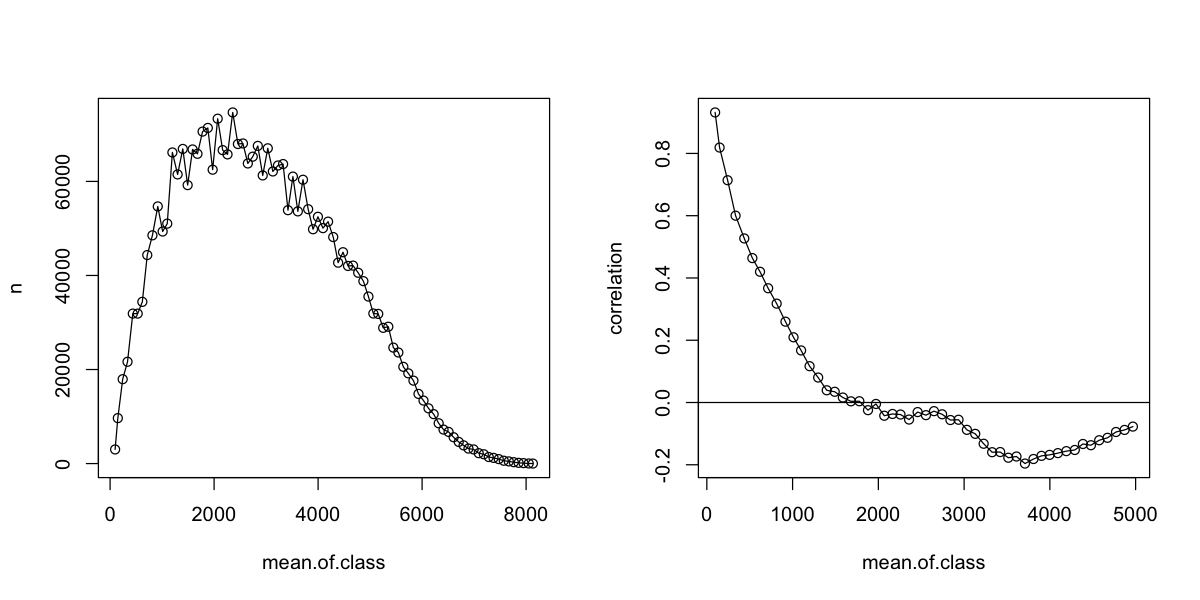
We can get more control on that plot by turning the object into a data frame. First, we can see that the number of pairs in a class drops off dramatically at large distances: that upswing on the right is based on few pairs, so we can generally ignore it and look at just shorter distances.
par(mfrow=c(1,2))
# convert three key variables into a data frame
rich.correlog <- data.frame(rich.correlog[1:3])
# plot the size of the distance bins
plot(n ~ mean.of.class, data=rich.correlog, type='o')
# plot a correlogram for shorter distances
plot(correlation ~ mean.of.class, data=rich.correlog, type='o', subset=mean.of.class < 5000)
# add a horizontal zero correlation line
abline(h=0)
One key use of correlograms is to assess how well models have controlled for spatial autocorrelation by looking at the correlation in the residuals. We can compare our two models like this and see how much better the SAR is at controlling for the autocorrelation in the data.
# Calculate correlograms for the residuals in the two models
simple.correlog <- with(data_sf, correlog(x, y, simple_resid, increment=cellsize, resamp=0))
sar.correlog <- with(data_sf, correlog(x, y, sar_resid, increment=cellsize, resamp=0))
# Convert those to make them easier to plot
simple.correlog <- data.frame(simple.correlog[1:3])
sar.correlog <- data.frame(sar.correlog[1:3])
# plot a correlogram for shorter distances
plot(correlation ~ mean.of.class, data=simple.correlog, type='o',
subset=mean.of.class < 5000)
# add the data for the SAR model to compare them
lines(correlation ~ mean.of.class, data=sar.correlog, type='o',
subset=mean.of.class < 5000, col='red')
# add a horizontal zero correlation line
abline(h=0)
Generalised least squares#
Generalised least squares (GLS) is an another extension of the linear model framework that allows us to include the expected correlation between our data points in the model fitting. The immediate way it differs from spatial autoregressive models is that it does not use a list of cell neighbourhoods, instead it uses a mathematical function to describe a model of how correlation changes with distance.
The gls function works in the same way as lm but permits extra arguments, so we can
fit the simple model:
gls_simple <- gls(avian_richness ~ mean_aet + mean_temp + elev, data=data_sf)
summary(gls_simple)
Generalized least squares fit by REML
Model: avian_richness ~ mean_aet + mean_temp + elev
Data: data_sf
AIC BIC logLik
28858.04 28887.11 -14424.02
Coefficients:
Value Std.Error t-value p-value
(Intercept) 198.27669 21.062878 9.41356 0
mean_aet 0.17839 0.004665 38.23824 0
mean_temp -4.50836 0.713118 -6.32204 0
elev 0.07360 0.005422 13.57341 0
Correlation:
(Intr) mean_t mn_tmp
mean_aet -0.315
mean_temp -0.975 0.148
elev -0.821 0.184 0.753
Standardized residuals:
Min Q1 Med Q3 Max
-3.5303243 -0.6523947 -0.0187891 0.6394739 3.9951462
Residual standard error: 81.24335
Degrees of freedom: 2479 total; 2475 residual
That looks very like the normal lm output - and indeed the coefficients are identical
to the same model fitted using lm above. One difference is gls output includes a
matrix showing the correlations between the explanatory variables. If I was being
critical, I would say that elevation and temperature are a rather highly correlated and
that multicollearity might be a problem (see
Practical 2).
To add spatial autocorrelation into the model we have to create a spatial correlation
structure. The example below uses the Gaussian model. You can look at ?corGaus for the
equation but essentially this model describes how the expected correlation decreases
from an initial value with increasing distance until a range threshold is met - after
that the data is expected to be uncorrelated. The constructor needs to know the spatial
coordinates of the data (using form) and then the other arguments set the shape of the
curve. You can set fixed=FALSE and the model will then try and optimise the range and
nugget parameters, but this can take hours.
# Define the correlation structure
cor_struct_gauss <- corGaus(value=c(range=650, nugget=0.1), form=~ x + y, fixed=TRUE, nugget=TRUE)
# Add that to the simple model - this might take a few minutes to run!
gls_gauss <- update(gls_simple, corr=cor_struct_gauss)
summary(gls_gauss)
Generalized least squares fit by REML
Model: avian_richness ~ mean_aet + mean_temp + elev
Data: data_sf
AIC BIC logLik
24586.56 24615.63 -12288.28
Correlation Structure: Gaussian spatial correlation
Formula: ~x + y
Parameter estimate(s):
range nugget
650.0 0.1
Coefficients:
Value Std.Error t-value p-value
(Intercept) 357.1643 46.27826 7.717755 0.000
mean_aet 0.0533 0.00851 6.257810 0.000
mean_temp -7.8843 1.60945 -4.898760 0.000
elev 0.0106 0.00929 1.141019 0.254
Correlation:
(Intr) mean_t mn_tmp
mean_aet -0.165
mean_temp -0.926 0.045
elev -0.878 0.076 0.935
Standardized residuals:
Min Q1 Med Q3 Max
-2.0344981 -0.0253260 0.6173762 1.1890697 4.3973486
Residual standard error: 96.62373
Degrees of freedom: 2479 total; 2475 residual
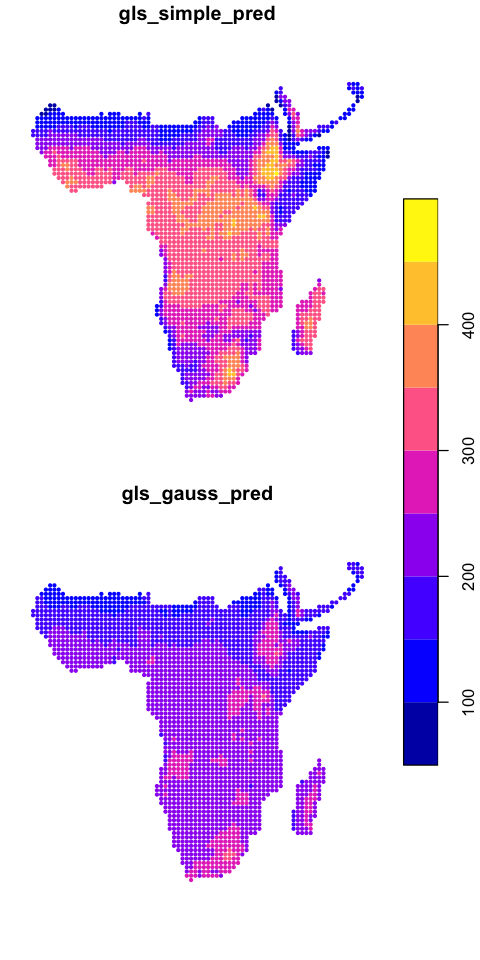
That output looks very similar but now includes a description of the correlation structure. Note that all of the model coefficients have changed and the significance of the variables has changed. Elevation is no longer significant when we account for autocorrelation.
We can map the two model predictions
data_sf$gls_simple_pred <- predict(gls_simple)
data_sf$gls_gauss_pred <- predict(gls_gauss)
plot(data_sf[c('gls_simple_pred','gls_gauss_pred')], key.pos=4, cex=0.6, pch=20)
Compare the residual autocorrelation
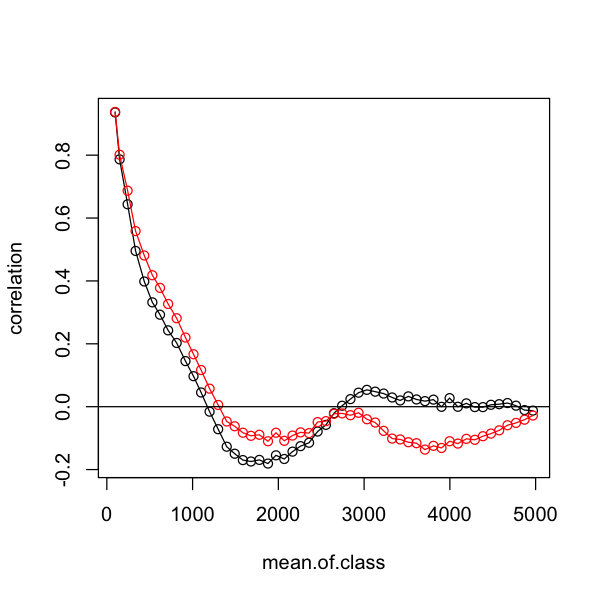
Using the example at the end of the section on autoregressive models, create this plot comparing the autocorrelation in the residuals from the two GLS models.
Show code cell source
#Extract the residuals
data_sf$gls_simple_resid <- resid(gls_simple)
data_sf$gls_gauss_resid <- resid(gls_gauss)
# Calculate correlograms for the residuals in the two models
simple.correlog <- with(data_sf,
correlog(x, y, gls_simple_resid,
increment=cellsize, resamp=0))
gauss.correlog <- with(data_sf,
correlog(x, y, gls_gauss_resid,
increment=cellsize, resamp=0))
# Convert those to make them easier to plot
simple.correlog <- data.frame(simple.correlog[1:3])
gauss.correlog <- data.frame(gauss.correlog[1:3])
# plot a correlogram for shorter distances
plot(correlation ~ mean.of.class, data=simple.correlog, type='o',
subset=mean.of.class < 5000)
# add the data for the SAR model to compare them
lines(correlation ~ mean.of.class, data=gauss.correlog, type='o',
subset=mean.of.class < 5000, col='red')
# add a horizontal zero correlation line
abline(h=0)
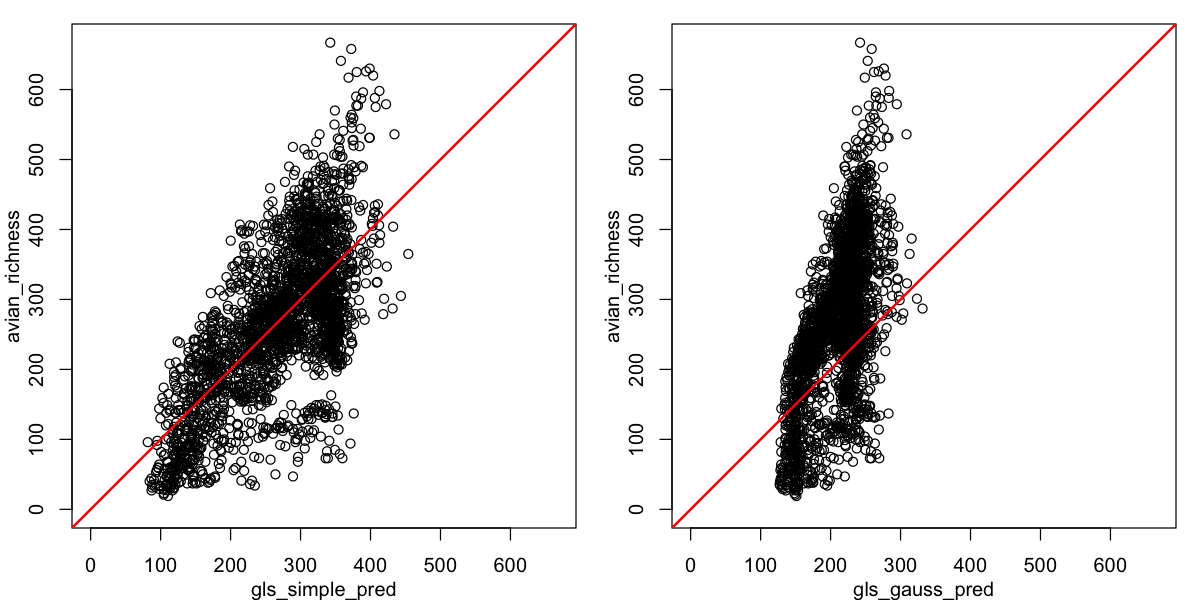
Oh dear - that is not the improvement we were looking for. We can also look at the relationship between observed and predicted richness. It is clear that - although it may be dealing with spatial autocorrelation - this spatial GLS is not a good description of the data! Do not forget that all models require careful model criticism!
par(mfrow=c(1,2), mar=c(3,3,1,1), mgp=c(2,1,0))
# set the plot limits to show the scaling clearly
lims <- c(0, max(data_sf$avian_richness))
plot(avian_richness ~ gls_simple_pred, data=data_sf, xlim=lims, ylim=lims)
abline(a=0, b=1, col='red', lwd=2)
plot(avian_richness ~ gls_gauss_pred, data=data_sf, xlim=lims, ylim=lims)
abline(a=0, b=1, col='red', lwd=2)
Eigenvector filtering#
This is yet another approach to incorporating spatial structure. In the example below,
we take a set of neighbourhood weights and convert them into eigenvector filters using
the meigen function from spmoran. Again, the package vignette is a great place for
further data (although the vignette name is a bit unhelpfully generic!)
vignette('vignettes', package='spmoran')
packageVersion('spmoran')
[1] ‘0.2.2.9’
In order to use this, we first need to extract the eigenvectors from our neighbourhood list. We need a different format, so we need to recreate the initial neighbours and then convert those to a weights matrix rather than sets of neighbour weights.
# Get the neighbours
queen <- dnearneigh(data_sf, d1=0, d2=sqrt(2) * cellsize + 1)
# Convert to eigenvector filters - this is a bit slow
queen_eigen <- meigen(cmat = nb2mat(queen, style='W'))
Note: cmat is symmetrized by ( cmat + t( cmat ) ) / 2
836/2479 eigen-pairs are extracted
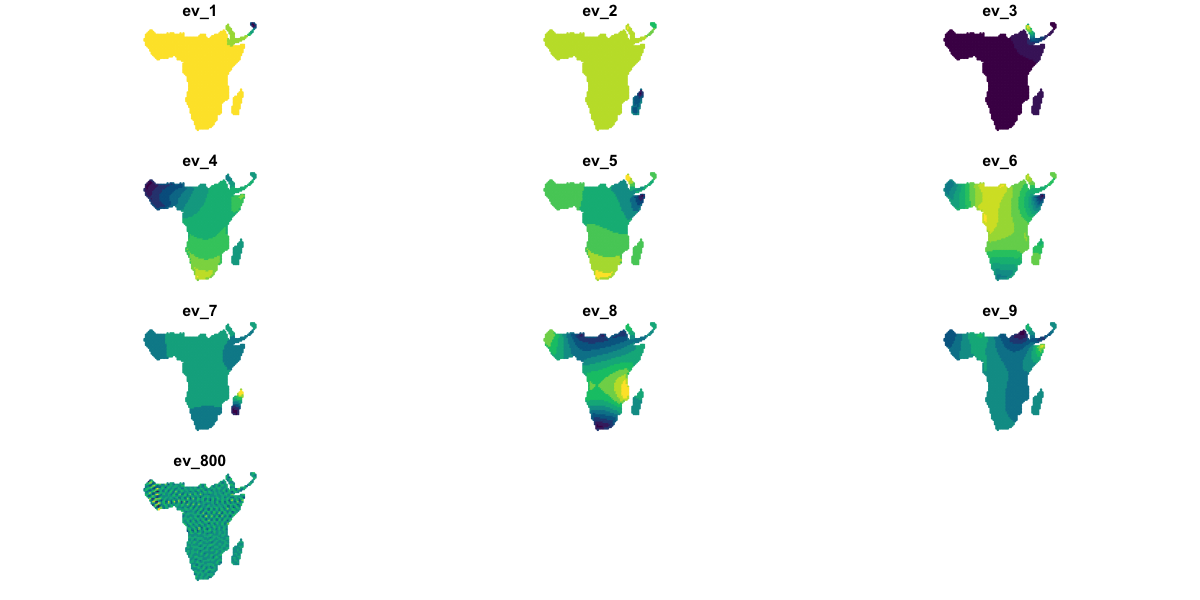
The queen_eigen object contains a matrix sf of spatial filters (not the same as the
sf data frame class!) and a vector ev of eigenvalues. Each column in
queen_eigen$sf shows a different pattern in the spatial structure and has a value for
each data point; the corresponding eigenvalue is a measure of the strength of that
pattern.
We’ll look at some examples:
# Convert the `sf` matrix of eigenvectors into a data frame
queen_eigen_filters <- data.frame(queen_eigen$sf)
names(queen_eigen_filters) <- paste0('ev_', 1:ncol(queen_eigen_filters))
# Combine this with the model data frame - note this approach
# for combining an sf dataframe and another dataframe by rows
data_sf <- st_sf(data.frame(data_sf, queen_eigen_filters))
# Visualise 9 of the strongest filters and 1 weaker one for comparison
plot(data_sf[c('ev_1', 'ev_2', 'ev_3', 'ev_4', 'ev_5', 'ev_6',
'ev_7', 'ev_8', 'ev_9', 'ev_800')],
pal=hcl.colors, max.plot=10, cex=0.2)
The spmoran package provides a set of more sophisticated (and time-consuming)
approaches but here we are simply going to add some eigenvector filters to a standard
linear model. We will use the first 9 eigenvectors.
eigen_mod <- lm(avian_richness ~ mean_aet + elev + mean_temp + ev_1 + ev_2 +
ev_3 + ev_4 + ev_5 + ev_6 + ev_7 + ev_8 + ev_9, data=data_sf)
summary(eigen_mod)
Call:
lm(formula = avian_richness ~ mean_aet + elev + mean_temp + ev_1 +
ev_2 + ev_3 + ev_4 + ev_5 + ev_6 + ev_7 + ev_8 + ev_9, data = data_sf)
Residuals:
Min 1Q Median 3Q Max
-186.443 -49.031 0.535 48.127 315.401
Coefficients:
Estimate Std. Error t value Pr(>|t|)
(Intercept) 2.506e+02 3.536e+01 7.086 1.79e-12 ***
mean_aet 1.443e-01 5.567e-03 25.917 < 2e-16 ***
elev 5.575e-02 6.288e-03 8.867 < 2e-16 ***
mean_temp -5.142e+00 1.227e+00 -4.190 2.89e-05 ***
ev_1 3.781e+02 7.007e+01 5.396 7.48e-08 ***
ev_2 1.560e+03 7.314e+01 21.333 < 2e-16 ***
ev_3 -1.056e+03 7.256e+01 -14.558 < 2e-16 ***
ev_4 1.997e+02 1.182e+02 1.690 0.0912 .
ev_5 1.557e+02 1.032e+02 1.509 0.1315
ev_6 1.916e+02 9.099e+01 2.106 0.0353 *
ev_7 1.508e+02 7.029e+01 2.146 0.0320 *
ev_8 1.166e+03 7.479e+01 15.585 < 2e-16 ***
ev_9 -3.158e+02 6.994e+01 -4.515 6.62e-06 ***
---
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1
Residual standard error: 68.23 on 2466 degrees of freedom
Multiple R-squared: 0.6251, Adjusted R-squared: 0.6232
F-statistic: 342.6 on 12 and 2466 DF, p-value: < 2.2e-16
In that output, we do not really care about the coefficients for each filter: those elements are just in the model to control for spatial autocorrelation. We might want to remove filters that are not significant.
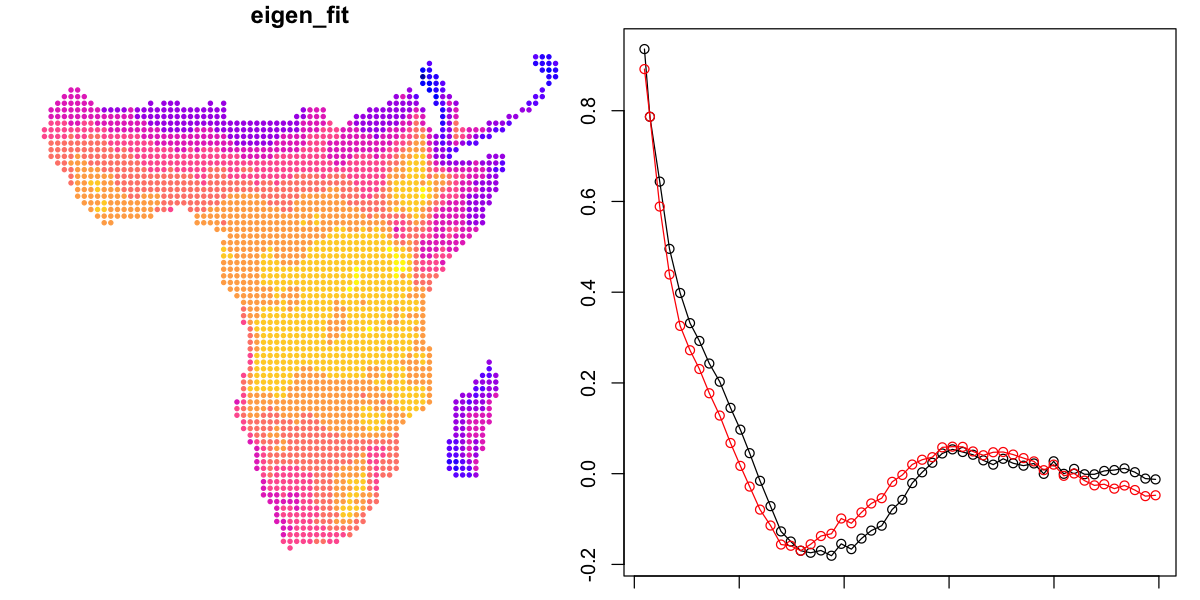
Using the tools above, we can look at the residual spatial autocorrelation and predictions from this model.
data_sf$eigen_fit <- predict(eigen_mod)
data_sf$eigen_resid <- resid(eigen_mod)
eigen.correlog <- with(data_sf, correlog(x, y, eigen_resid, increment=cellsize, resamp=0))
eigen.correlog <- data.frame(eigen.correlog[1:3])
par(mfrow=c(1,2))
plot(data_sf['eigen_fit'], pch=20, cex=0.7, key.pos=NULL, reset=FALSE)
# plot a correlogram for shorter distances
plot(correlation ~ mean.of.class, data=simple.correlog, type='o',
subset=mean.of.class < 5000)
# add the data for the SAR model to compare them
lines(correlation ~ mean.of.class, data=eigen.correlog, type='o',
subset=mean.of.class < 5000, col='red')
That is not much of an improvement either. To be fair to the spmoran package, the
approach is intended to consider a much larger set of filters and go through a selection
process. The modelling functions in spmoran do not use the formula interface - which
is a bit hardcore - but the code would be as follows. I do not recommend running
this now - I have no idea of how long it would take to finish but it is at least quarter
of an hour and might be days.
# Get a dataframe of the explanatory variables
expl <- data_sf[,c('elev','mean_temp','mean_aet')]
# This retains the geometry, so we need to convert back to a simple dataframe
st_geometry(expl) <- NULL
# Fit the model, optimising the AIC
eigen_spmoran <- esf(data_sf$avian_richness, expl, meig=queen_eigen, fn='aic')